Fri, Nov 29, 2024
[Archive]
Volume 19, Issue 3 (September 2022)
IJMSE 2022, 19(3): 1-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sharifi O, Golmohammad M, Soozandeh M, Oskouee M. Effects of Al Doping on the Properties of Li7La3Zr2O12 Garnet Solid Electrolyte Synthesized by Combustion Sol-gel Method. IJMSE 2022; 19 (3) :1-10
URL: http://ijmse.iust.ac.ir/article-1-2631-en.html
URL: http://ijmse.iust.ac.ir/article-1-2631-en.html
Abstract: (8926 Views)
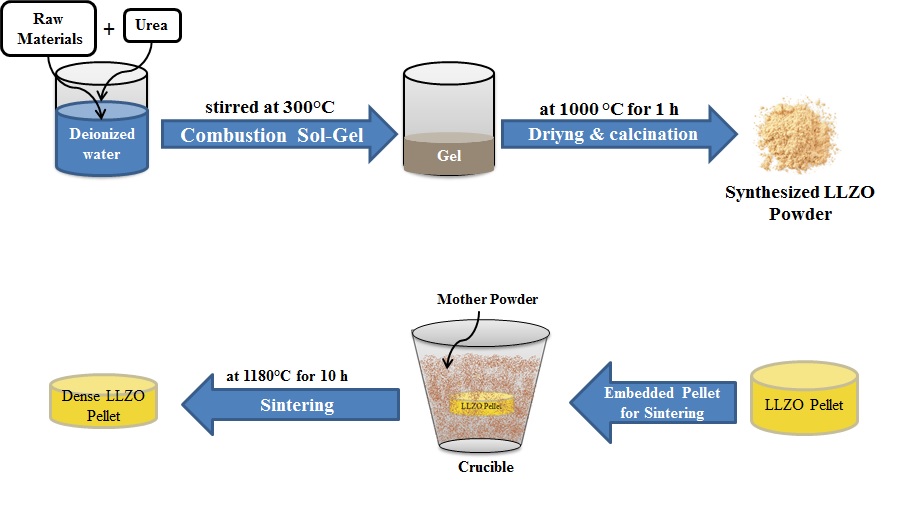
Li7La3Zr2O12 (LLZO) garnets are one of the promising materials as electrolytes for solid-state batteries. In this study, Li7-3xAlxLa3Zr2O12 (x= 0.22, 0.25, and 0.28) garnet is synthesized using the combustion sol-gel method to stabilize the cubic phase for higher ionic conductivity. The X-ray diffraction (XRD) results of as-synthesized powders reveal that by addition of 0.22 and 0.25 mole Al, the tetragonal phase still co-exist, whereas 0.28 mole Al addition resulted in a single cubic phase. Afterward, the as-synthesized powders are pressed and sintered at 1180°C for 10h. The hardness evaluation revealed that Al addition increases the hardness that shows better resistance against Li dendrite formation. Besides, the secondary electron microscopy results demonstrate that the dopant has not a huge impact on particle size and grain growth whereas the porosity content has been changed. Finally, the investigation of samples' electrochemical behavior reveals that the addition of Al increases the ionic conductivity of samples by increasing the density and stability of the cubic phase as well. The results declare that the 0.25 Al sample has the highest ionic conductivity. This behavior is thought to be due to the promotion of sintering and increment of bulk ionic conductivity by doping Al.
Keywords: Solid electrolyte, Combustion sol-gel, electrochemical characteristics, Li7La3Zr2O12 (LLZO), Al-doped
Type of Study: Research Paper |
Subject:
Ceramics
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |