BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmse.iust.ac.ir/article-1-918-en.html
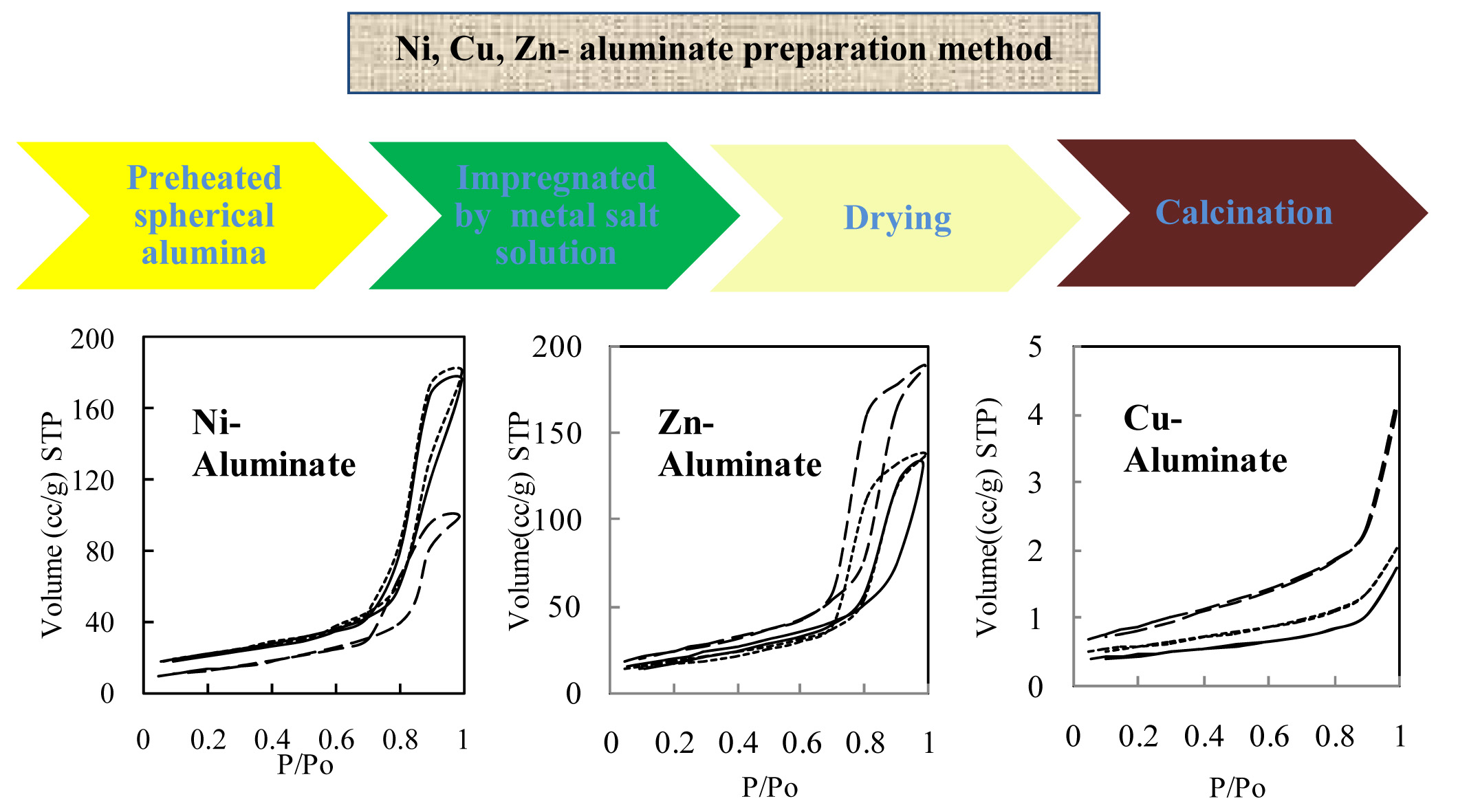
A series of MAl2O4 (M=Ni, Zn, and Cu) aluminates were prepared by using impregnation method; the metal content of the products was ranged between 5wt% to 25wt%. The samples were characterized by x-ray diffraction (XRD), Brunauer Emmett Teller (BET) surface area, NH3 temperature-programmed desorption (NH3-TPD), and inductively coupled argon plasma (ICP).
The specific surface areas of zinc, nickel and copper aluminates were in the ranges of 47-77m2/g, 63-87m2/g and 1.6-3m2/g, respectively. The surface acidity decreased in the order of CuAl2O4<< NiAl2O4< ZnAl2O4<< Al2O3. By increasing the amount of metals in the samples, the number of acidic sites decreased, but their strength did not significantly change. Ni-aluminates have fewer acidic sites than Zn-aluminates, particularly in strong acid sites
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |