Sat, Nov 23, 2024
[Archive]
Volume 16, Issue 4 (December 2019)
IJMSE 2019, 16(4): 1-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Palizdar M, Aslam Z, Aghababazadeh R, Mirhabibi A, Sangpour P, Abadi Z, et al . Chemical Interaction Between MgO Support and Iron Catalyst. IJMSE 2019; 16 (4) :1-9
URL: http://ijmse.iust.ac.ir/article-1-1253-en.html
URL: http://ijmse.iust.ac.ir/article-1-1253-en.html
M. Palizdar 
 , Z. Aslam
, Z. Aslam 
 , R. Aghababazadeh
, R. Aghababazadeh 
 , A. Mirhabibi
, A. Mirhabibi 
 , P. Sangpour
, P. Sangpour 
 , Z. Abadi
, Z. Abadi 
 , Y. Palizdar
, Y. Palizdar 
 , R. Brydson
, R. Brydson 


 , Z. Aslam
, Z. Aslam 
 , R. Aghababazadeh
, R. Aghababazadeh 
 , A. Mirhabibi
, A. Mirhabibi 
 , P. Sangpour
, P. Sangpour 
 , Z. Abadi
, Z. Abadi 
 , Y. Palizdar
, Y. Palizdar 
 , R. Brydson
, R. Brydson 

Abstract: (14607 Views)
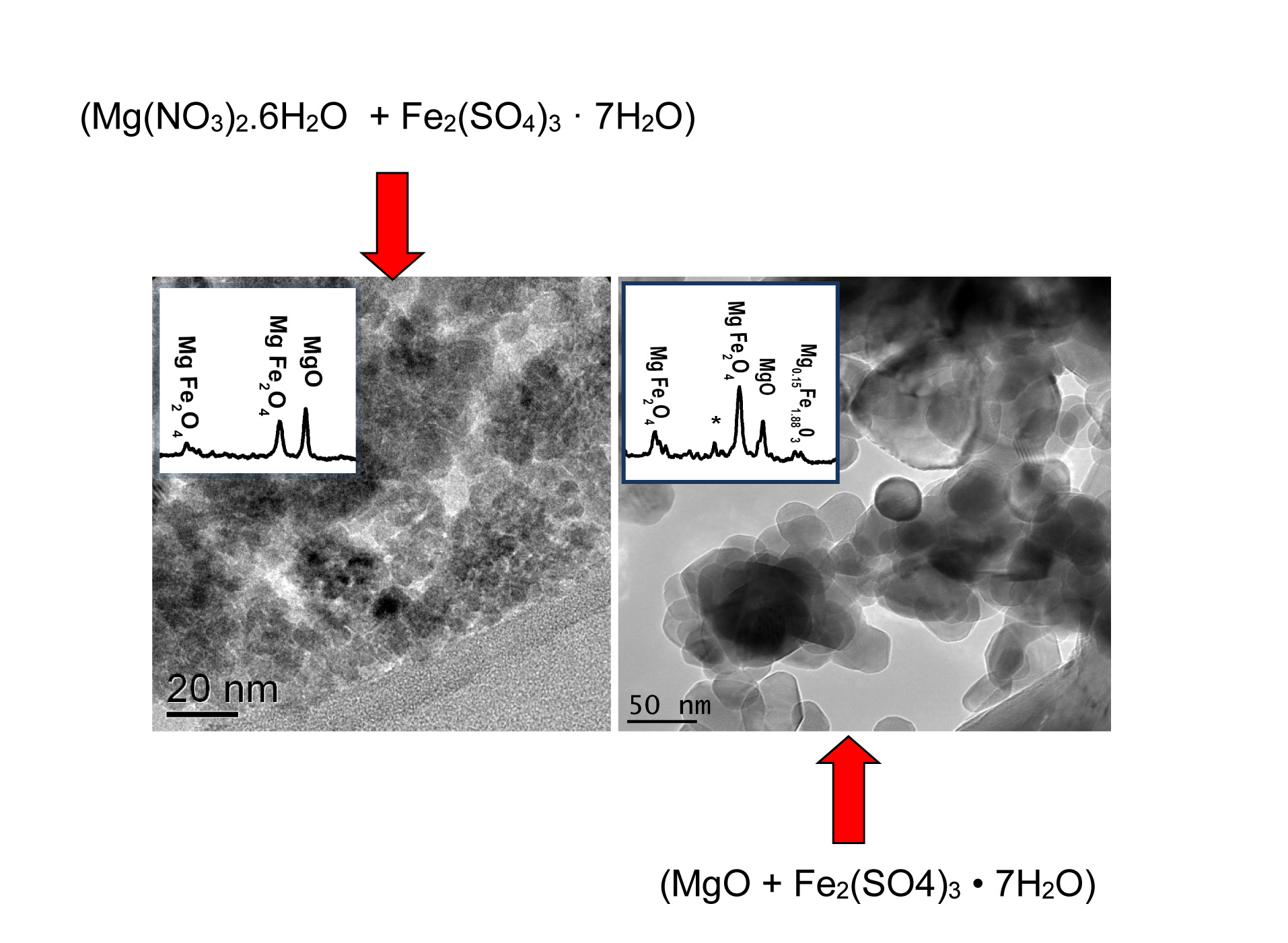
In this paper the chemical interaction between catalyst and support has been studied to understand the observed different growth rate of CNTs in our previous paper. Both pure MgO and Mg(NO3)2 . 6H2O as sources of the MgO catalyst support and Fe2(SO4)3 · xH2O as the source of the Fe catalyst, were employed. A Fe catalyst supported on MgO has been synthesized using the wet impregnation method followed by calcination. To compare the catalyst grain size and its distribution, the sample were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray powder diffraction (XRD) and BET specific surface area (SSA) measurement and X-ray photoelectron spectroscopy (XPS). XPS technique have utilized complementary to demonstrate the existence of chemical interaction between MgO support and Fe catalyst. Results revealed that the type of precursor used to prepare the support has a significant influence on the morphology of the support and the associated distribution of the Fe catalysts. The highest yield of MgFe2O4 phase was obtained using a pure MgO precursor which after calcination results in a homogenous distribution of nano-sized Fe particles over the support surface
Type of Study: Research Paper |
Subject:
Ceramics
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |