Wed, Apr 9, 2025
[Archive]
Volume 19, Issue 4 (Desember 2022)
IJMSE 2022, 19(4): 1-14 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
moazzami Y, Gharabaghi M, Shafaei Tonkaboni Z. Leaching Kinetics of Chalcopyrite Concentrate by Ionic Liquids. IJMSE 2022; 19 (4) :1-14
URL: http://ijmse.iust.ac.ir/article-1-2812-en.html
URL: http://ijmse.iust.ac.ir/article-1-2812-en.html
Abstract: (9786 Views)
Ionic liquids as green solvents with high thermal stability, recyclability, low flash point, and low vapor
pressure, have been considered as a viable alternative in hydrometallurgical processes. In this study the leaching
kinetics of chalcopyrite concentrate was investigated using 1-Butyl-3-methylimidazolium hydrogen sulfate
(BmimHSO4) as an acidic ionic liquid. The Effect of operational parameters, including temperature, BmimHSO4
concentration, H2O2 concentration, stirring speed, solid-to-liquid ratio, and particle size on the rate of copper
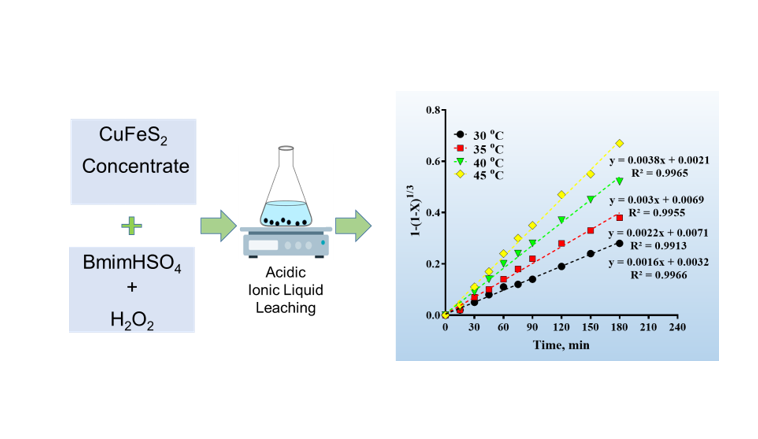
dissolution of CuFeS2 were examined systematically. The highest Cu efficiency (ca. 97%) was achieved using 40%
(w/v) BmimHSO4, 30 %v/v H2O2, and 10 g.L-1 solid to liquid ratio for particle sizes less than 37 μm at 300 rpm and
45°C after 180 min leaching time. Kinetics study using Shrinking Core Model (SCM) revealed that CuFeS2 leaching
process using BmimHSO4 follows chemical reaction-controlled process. Under these circumstances, the calculated
activation energy was 46.66 KJ/mol. Moreover, the orders of reaction with respect to BmimHSO4 and H2O2
concentration, solid to liquid ratio and particle size were estimated to be 0.539, 0.933, −0.676 and −1.101
respectively. The obtained Arrhenius constant was found to be 0.26 106. The calculation of apparent activation
energy using “time given to a fraction method” revealed that the leaching mechanism remains the same over the
course of time.
pressure, have been considered as a viable alternative in hydrometallurgical processes. In this study the leaching
kinetics of chalcopyrite concentrate was investigated using 1-Butyl-3-methylimidazolium hydrogen sulfate
(BmimHSO4) as an acidic ionic liquid. The Effect of operational parameters, including temperature, BmimHSO4
concentration, H2O2 concentration, stirring speed, solid-to-liquid ratio, and particle size on the rate of copper
dissolution of CuFeS2 were examined systematically. The highest Cu efficiency (ca. 97%) was achieved using 40%
(w/v) BmimHSO4, 30 %v/v H2O2, and 10 g.L-1 solid to liquid ratio for particle sizes less than 37 μm at 300 rpm and
45°C after 180 min leaching time. Kinetics study using Shrinking Core Model (SCM) revealed that CuFeS2 leaching
process using BmimHSO4 follows chemical reaction-controlled process. Under these circumstances, the calculated
activation energy was 46.66 KJ/mol. Moreover, the orders of reaction with respect to BmimHSO4 and H2O2
concentration, solid to liquid ratio and particle size were estimated to be 0.539, 0.933, −0.676 and −1.101
respectively. The obtained Arrhenius constant was found to be 0.26 106. The calculation of apparent activation
energy using “time given to a fraction method” revealed that the leaching mechanism remains the same over the
course of time.
Type of Study: Research Paper |
Subject:
Extractive Metallurgy
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |